Designing & developing a safer minimally invasive surgical access port.
www.xpanmedical.com
SUMMARY
A medical device 5+ years in the making.
At Toronto-based medical device startup, Xpan, I worked closely within a team of engineers, clinicians and industry leaders to bring a medical device from concept to fully-functioning, patented, manufactured and FDA-ready product.
Although my role in this project spanned product development, regulatory, quality, manufacturing, intellectual property, sales & marketing and beyond, for the purposes of this project outline, I will focus on my contributions to the design & development of the medical device.
My Role: R&D Engineer, Product Design & Development Lead
Timeline: Jan 2019 - Present
Tools Used: SolidWorks, FDM & SLA 3D printers, various hand tools (drill, rotary, saw), various measurement gauges (force, pressure)
Please note that many conceptual figures are not shown due to their proprietary nature.
PROBLEM
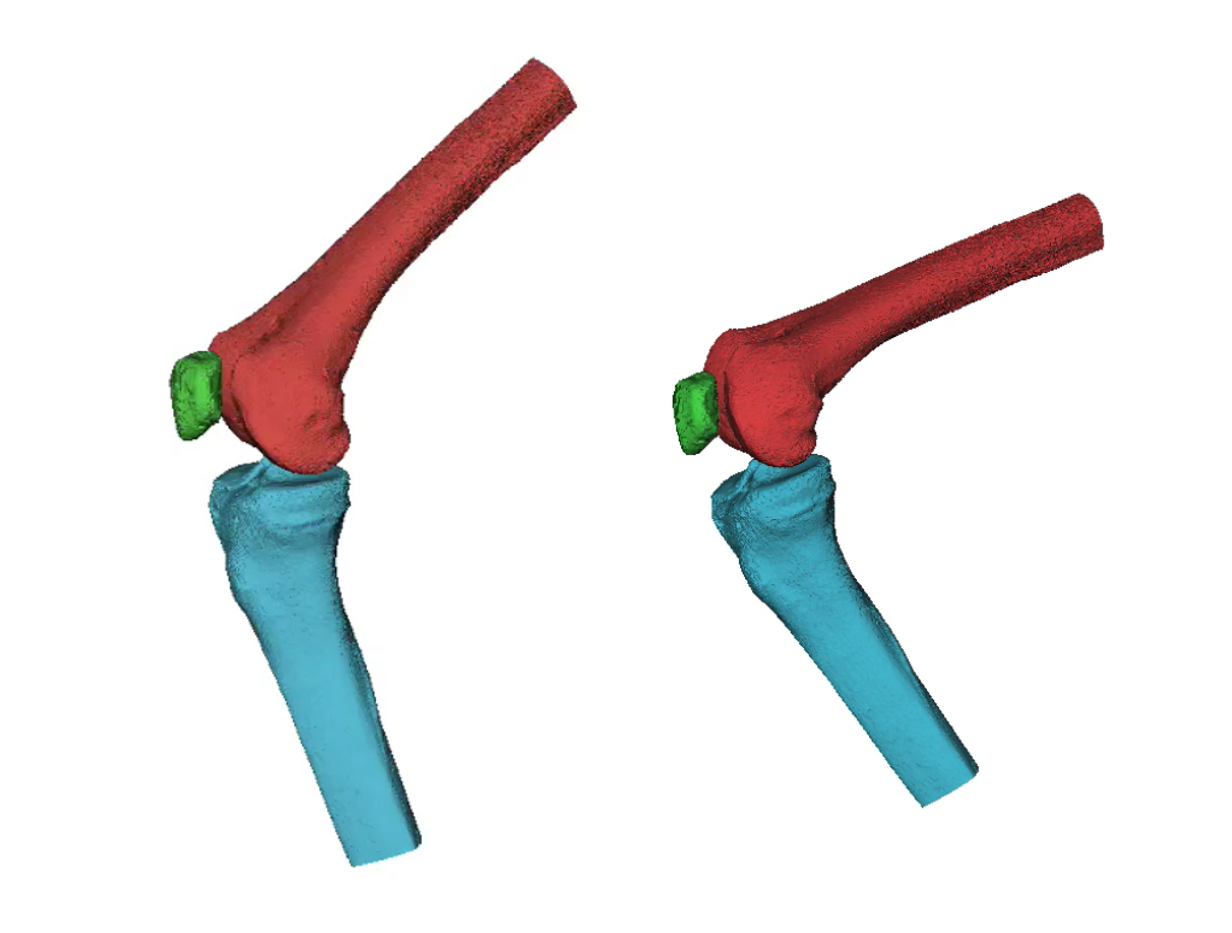
Minimally invasive surgery (laparoscopy) is performed through a series of incisions in a patient's abdomen, where cylindrical ports (called trocars) are inserted to create a tunnel for cameras and a variety of surgical instruments to perform the surgery.
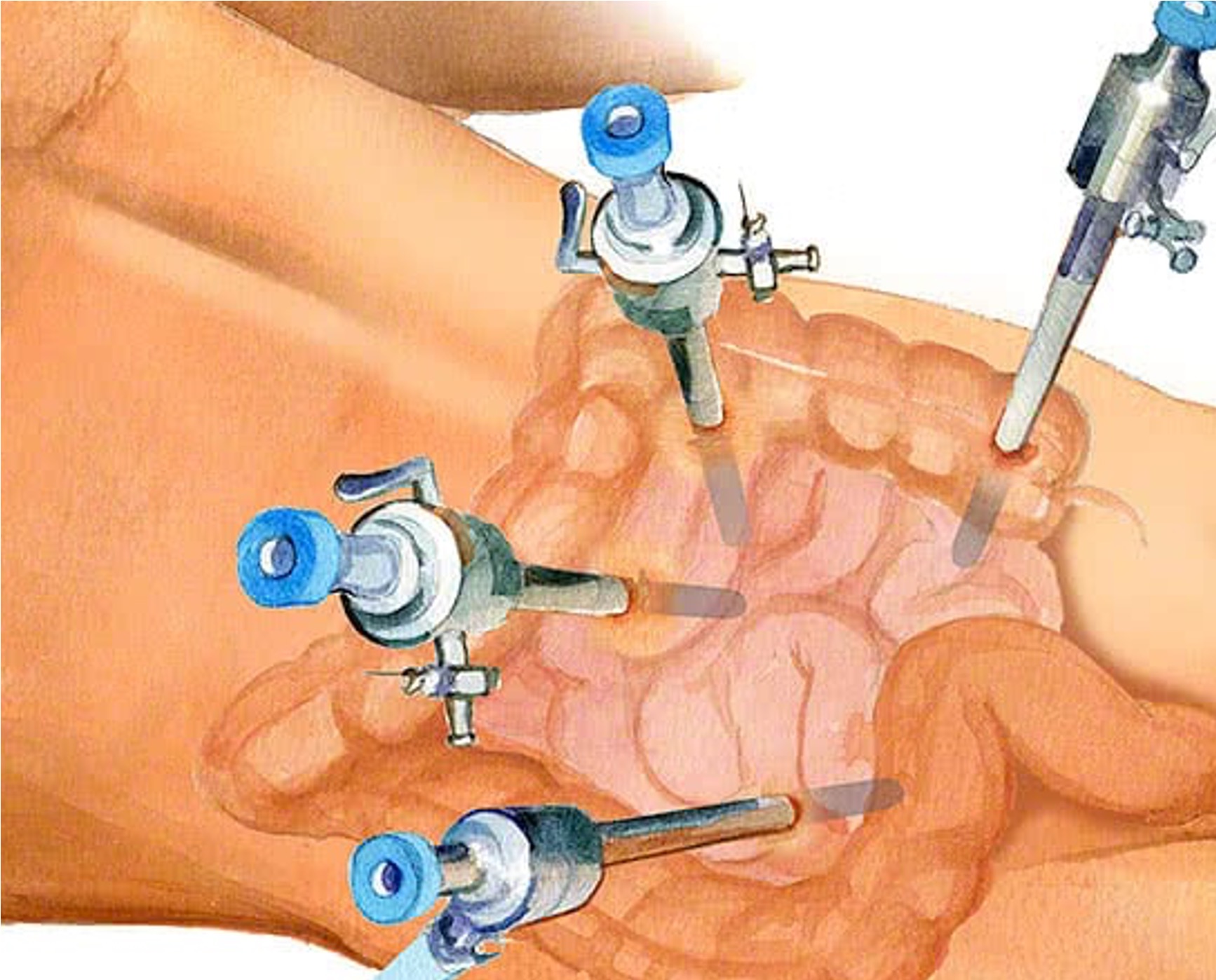
Trocar placement in the abdomen
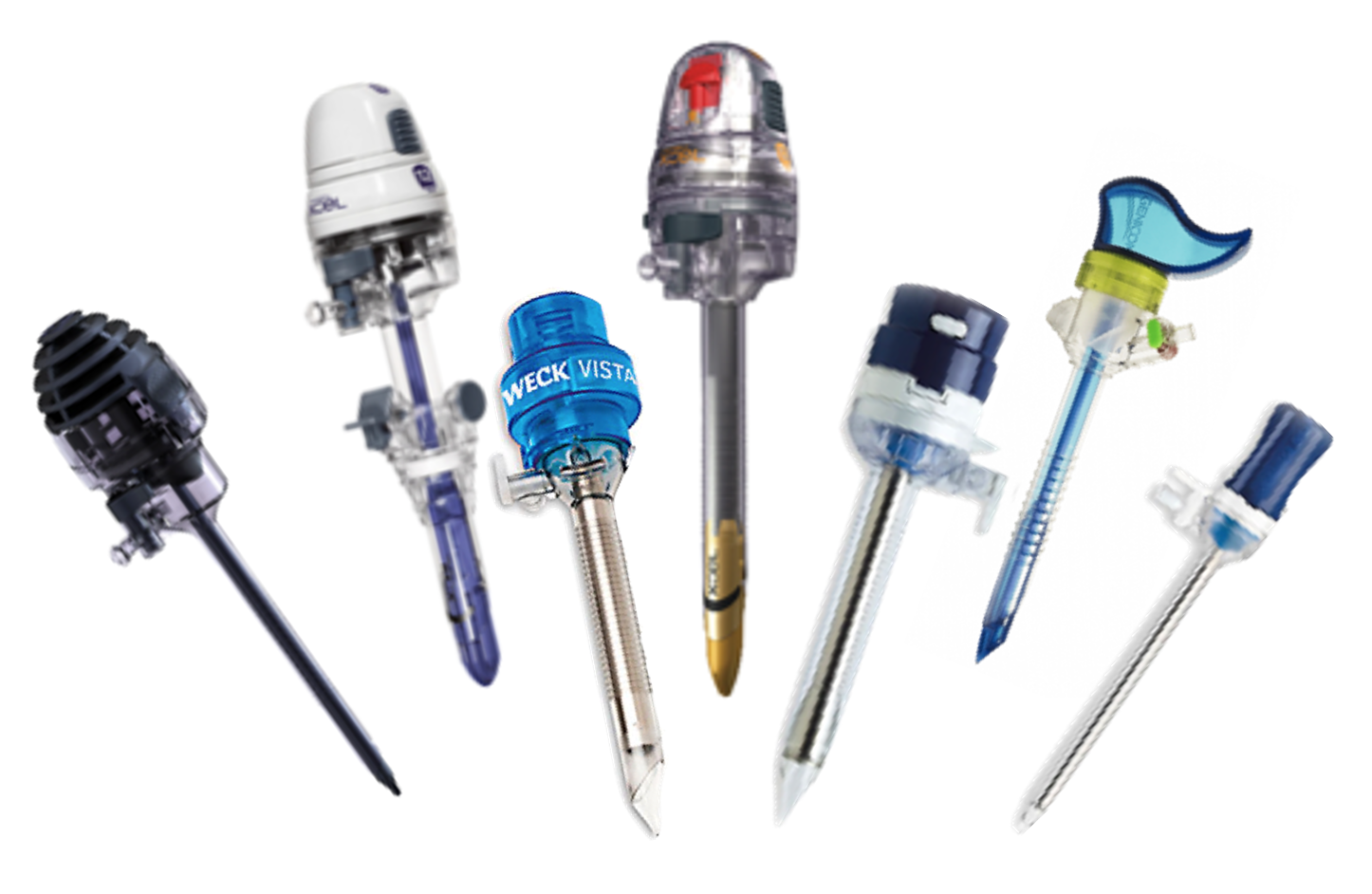
Common trocars
While instrumentation has improved over time, trocar technology has remained essentially the same for the past 30 years; they are fixed-diameter (3-15mm) ports. Some of the major trocar-related problems include:
- Excessive force upon entry can cause 50% of patient injuries
- Conventional large diameter (12-15mm) trocars cause worse scarring, post-operative pain and a higher risk of port-site hernias
- Lack of standardized trocar options and entry techniques
- Current intraprocedural trocar "upsizing" to larger diameters to fit larger instruments introduces patient risk and disrupts surgical workflow
- Frequent trocar slippage introduces operating room disruptions and inefficiencies
- Limited instrumentation options due to fixed diameter trocars
EXISTING SOLUTIONS
Trocars range from 3mm to 15mm in diameter and have various entry tip and cannula designs. Each existing design solves a specific problem, while compromising another. For example, a 'bladed tip' might reduce forces upon entry that a 'blunt tip' would otherwise cause, however this increases the risk of penetrating further and damaging tissue or internal organs. A user must compromise one solution over the next, however this should not be the case.
A summary of the advantages and distadvantages to each main trocar design category are as follows:
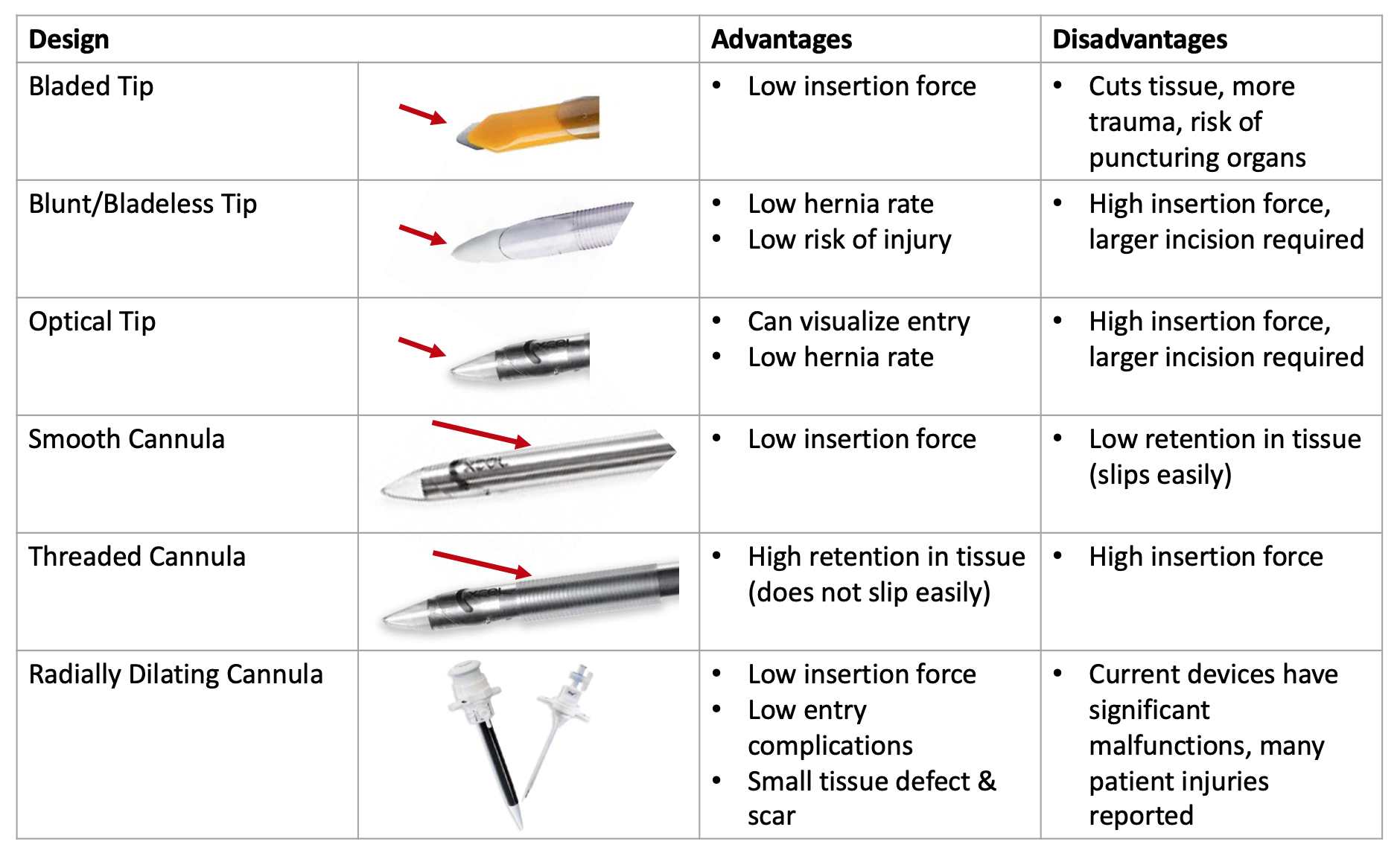
Major trocar design advantages and disadvantages summarized
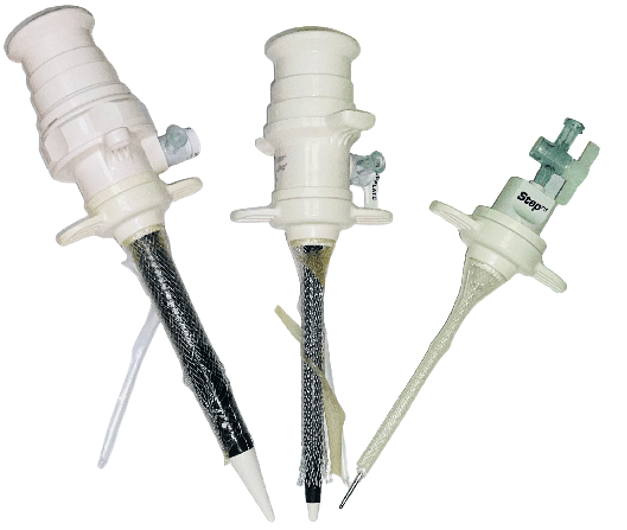
VersaStep radially dilating trocar in 12mm, 5mm and needle with mesh sleeve.
The VersaStep radially dilating trocar was introduced in the 90's as a safer alternative to fixed-diameter trocars. A Veress needle + mesh sleeve is inserted into the abdomen, followed by expanding the mesh sleeve using a 5mm or 12mm dilator, as shown on the left.
Literature comparing radially dilating to fixed diameter trocars indicates that radially dilating trocars reduce laparoscopic complications, create smaller fascial defects and improve patient reported outcomes such as post operative pain and return to work [1]. Despite the benefits of radial dilation, the VersaStep has not been adopted for widespread use due to substantial device malfunctions and usability concerns. Malfunctions such as the mesh sleeve breaking, and needle poking through the side of the mesh sleeve are documented in the FDA MAUDE database.
The VersaStep device is Xpan's FDA predicate, as it is the only other expandable trocar on the market.
OUR SOLUTION
Our goal was to develop a solution that would combat common user problems and patient-related issues, while also also preventing the compromise that otherwise occurs with fixed-diameter trocars, as outlined above.
Animation created in collaboration with a local contract medical animator.
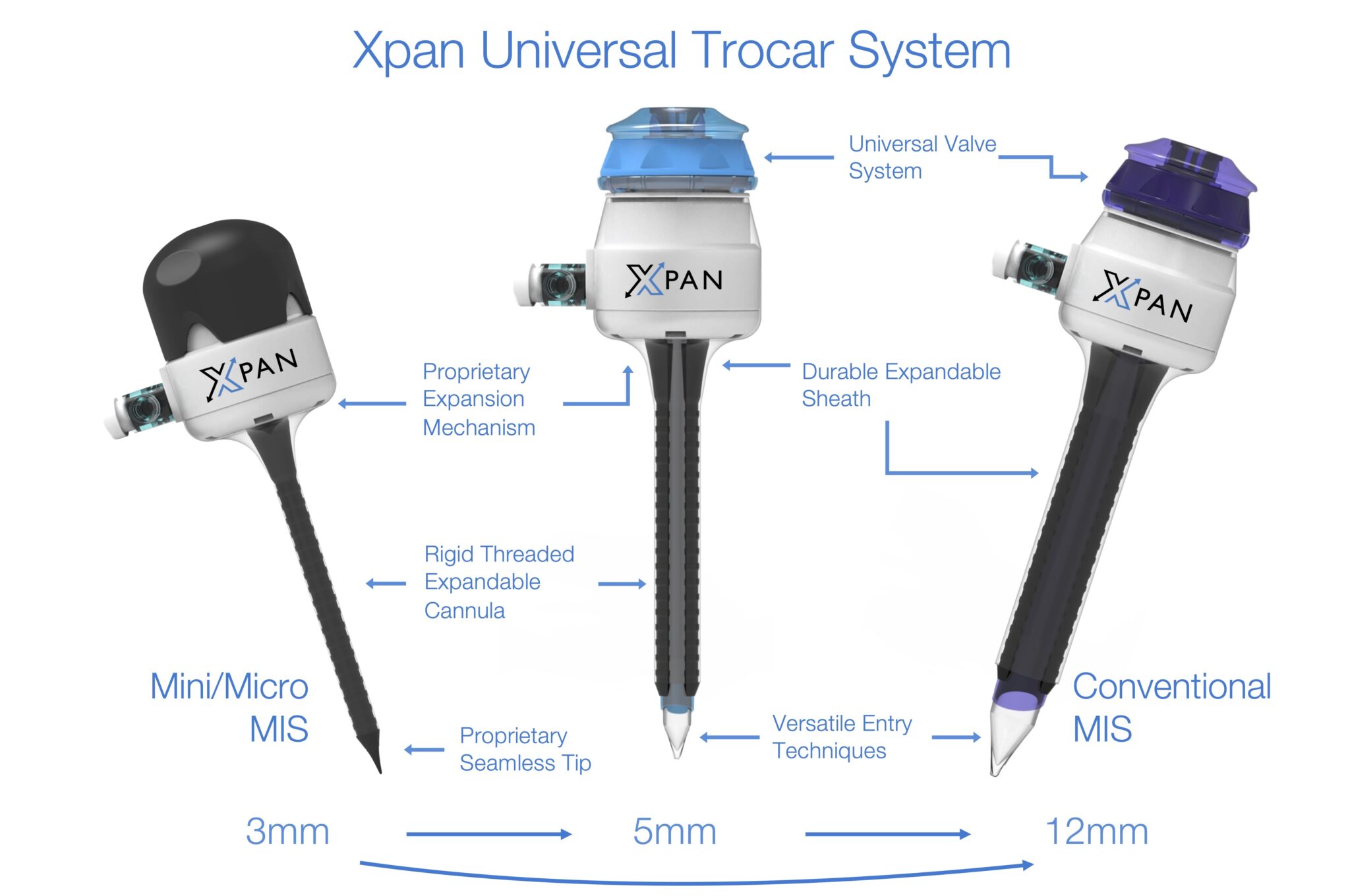
A smaller, less invasive and expandable surgical access port (trocar) that aims to reduce trauma to the patient, improve surgical flexibility and minimize operational and inventory inefficiencies.
Reducing Patient Trauma: A surgeon can enter the device in the abdomen at a miniature 3mm size with a blunt tip, significantly reducing the force of entry. They can then can expand the device up to 4x in diameter, stretching tissue instead of cutting it. The trocar's expansion and ribbed cannula provide extra retention in tissue.
Improving Surgical Flexibility: The multi size offerings allow surgeons to "up-size" trocars during surgery to fit larger instruments, providing them with additional intraoperative flexiblity, while maintaining insufflation levels (gas in the abdomen to create a larger working space). Surgeons also have the option of inserting the trocar optically, if that method is preferred.
Optimizing Hospital Inventory: Having a universal, 3-in-1 size trocar with different entry options (3mm blunt, 5mm and 12mm optical) has the potential reduce a hospital's trocar SKUs significantly, minimizing inventory headaches. Instead of having to carry 3mm, 5mm and 12mm trocars with various tip designs (bladed, optical, etc.) and cannula designs (threaded, smooth, etc.), they can carry one device in stock.
More details on the solution can be found on the Xpan website.
Due to the ongoing nature of FDA applications, I cannot disclose specific quantitative outcomes related to the performance of the device and/or clinical use cases.
The Xpan Universal Trocar System has not received regulatory approval or clearance in any jurisdiction and is not for sale. Details about the Xpan Universal Trocar System are being provided as general company information only and should not be construed as product or service advertisement. Expected FDA clearance & commercial release early 2023.
PROCESS
As a startup in a fast-paced environment where priorities constantly shift, our design and development process was not exactly "linear". However, over the span of 4+ years, much of our process was influenced by Stanford University Biodesign and Starfish Medical Device Commercialization principles. Xpan's medical device design & development process loosely followed these phases:
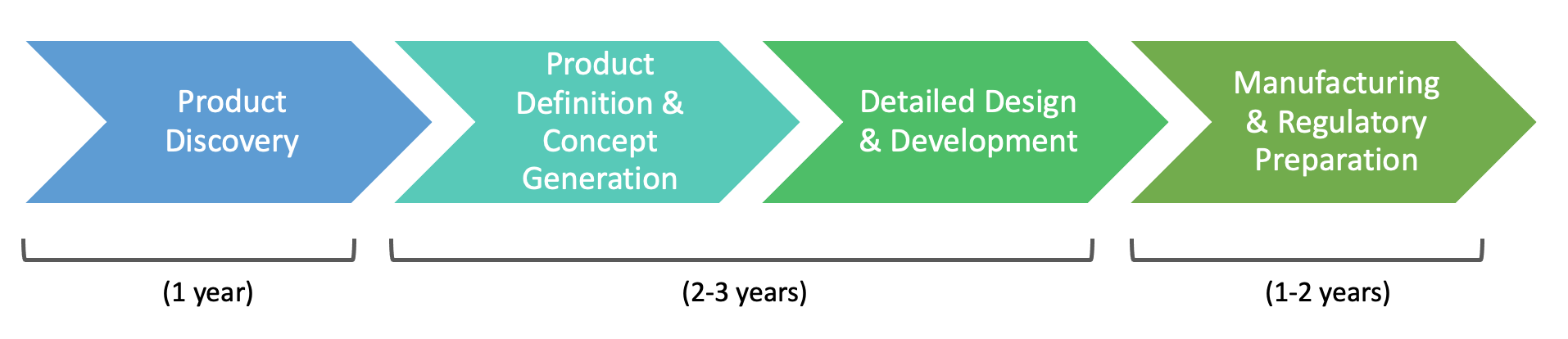
1. Product Discovery
I joined the team after the Product Discovery phase. It was spun out of the founder, Zaid Atto's university capstone project, where he worked directly with pediatric surgeons in Toronto to identify needs and pursue this solution. During this time, Zaid developed proof-of-concept prototypes, conducted initial bench tests, interviewed end-users, submitted a provisional patent application and began officially building the company.
2. Product Definition & Concept Generation
I was brought onto the Xpan team during the Product Definiton phase, where I:
- Developed a list of functional requirements (e.g. Trocar must be able to fit instrument sizes 3-12mm, Trocar must prevent gas leakage with and without instrument manipulation within the valves)
- Developed a list of usability requirements (e.g. Trocar body must be easy to hold, Trocar body must not be top heavy or interfere with surrounding trocars)
- Conducted literature research on complications, researched competitor devices and existing solutions
- Sketched 50+ design concepts for parts of the product that were not fully fleshed out
- Conducted FTO IP searches for new design concepts
- Created 3D printed 30+ prototypes
- Created an FMEA to assess risk
- Created testing jigs and conducted bench tests to assess the functional requirements (e.g. to measure the force of insertion, removal and expansion in tissue, instrument drag forces and gas leakage)
- Sent surveys and interviewed 50+ end-users, shared prototypes with them to get early usability feedback
My device design concepts are proprietary and cannot be shown, however I have included some non-proprietary testing jig design sketches:
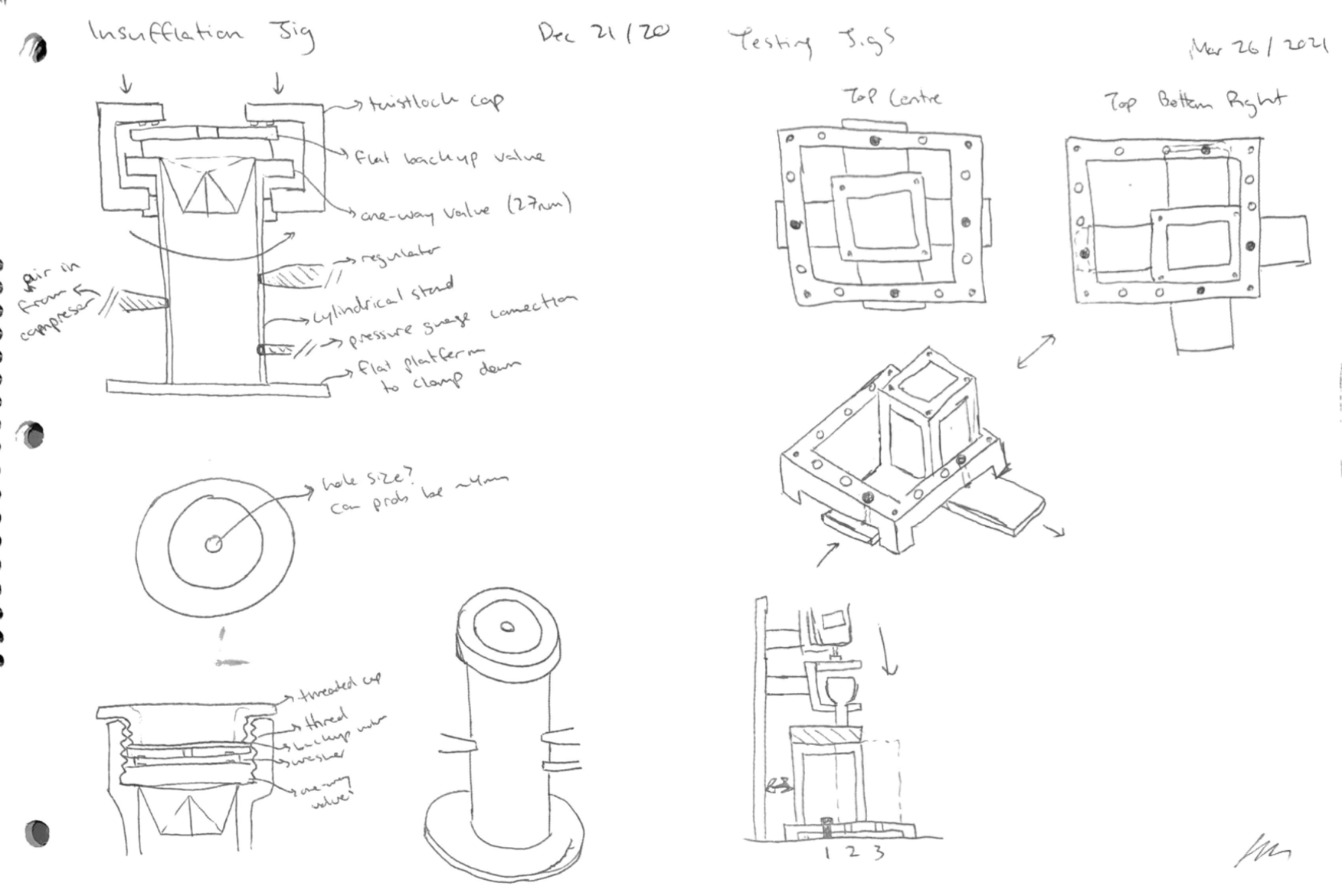
Testing jig design sketches. Left: testing valve gas leakage. Right: a modular force stand for testing insertion/removal and expansion forces in tissue.
One of my first and largest design challenges was to optimize the size and weight of the entire device, considering CAD and material changes. This took 10+ SolidWorks iterations, where I had to optimize the angle of the strut + cam mechanism and reduce the overall height of the valves, while retaining functionality. Some design changes I included were reducing the 'struts' (the prongs/fingers on the trocar that expand) from 4 to 3, changing stainless steel materials to machined plastic, and eliminating unecessary material that didn't affect functionality.
I successfully reduced the height by approximately 40%, diameter by 10% and weight by 25%. See various trocar prototypes and height optimization over time:
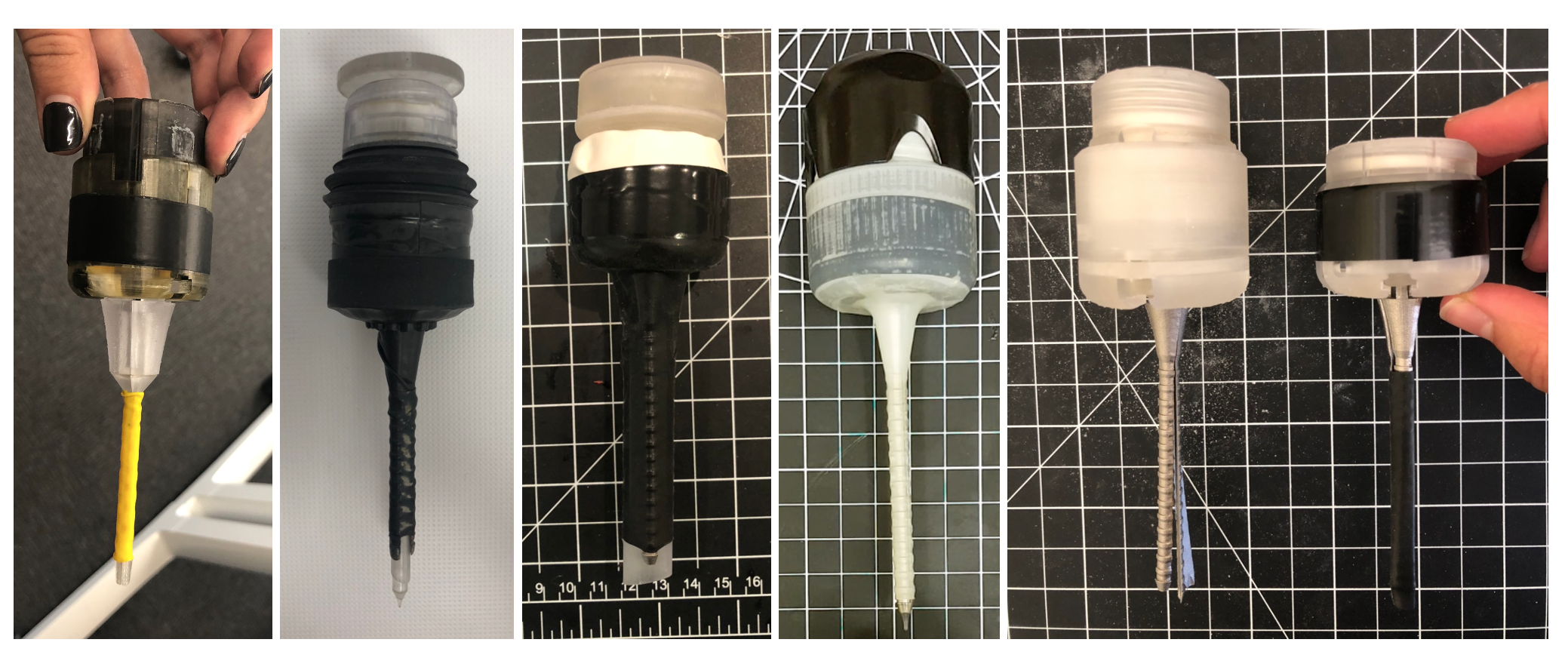
Xpan early trocar prototype evolution
3. Detailed Design & Development
This phase brought the device from low fidelity to high fidelity, ironing out any functional or usability issues and ensuring the device met requirements. During this process, I:
- Developed high quality CAD
- Further optimized device size & ergonomics
- Sourced FDA-approved materials and suppliers to be used; e.g. suppliers capable of injection and dip molding for the elastomeric parts (valves and expandable cover)
- Troubleshooted design issues
- e.g. Since our expandable port expands from 3mm-12mm, the valve system had to allow for instruments in that size range to not only fit and not rip, but also have low drag forces. Our original material was not condusive to these requirements; our drag forces were higher than competitors and the valve ripped with sharper instruments. We explored several lubricants, chemical coating, and the addition of a 'protective shield' to prevent rippage.
- Improved bench testing jigs, collected high quality data with competitor devices in preparation for V&V tests
- Tested device in a simulated surgical environment; 4 pre-clinical (animal) studies were conducted at Canadian & US hospitals
- Conducted 30+ user interviews throughout the process

5mm cannula valve

Devices laid out before a pre-clinical study
4. Manufacturing, Regulatory Preparation
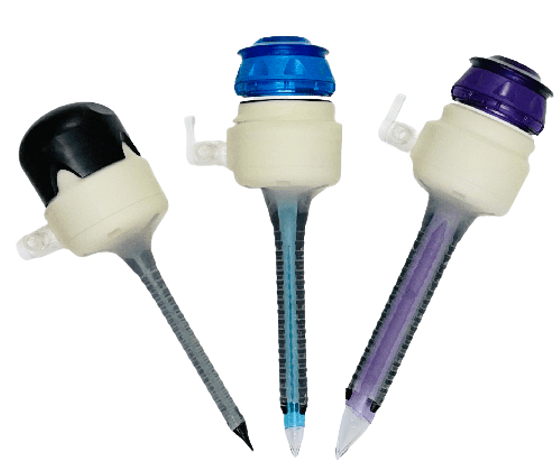
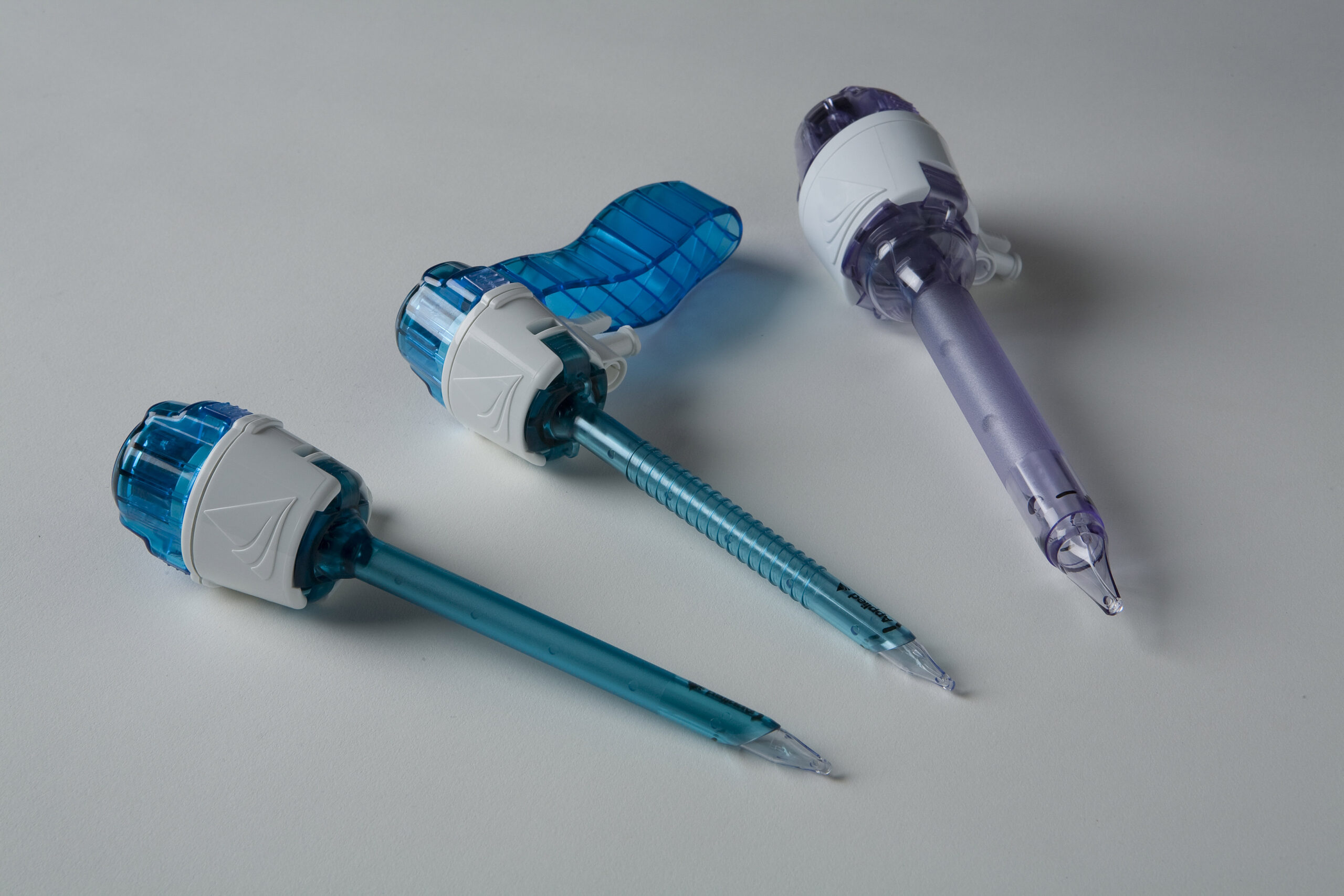
Manufactured device in 3mm, expanded 5mm and expanded 12mm configurations
We initiated conversations with a medical device contract manufacturer in New Hampshire. Now that we had a high fidelity prototype, we needed to make sure each part was manufacturable. I worked directly with the CM on design for manufacturing (DFM), turning 3D-printable and CNC parts into injection-moldable parts. For example, this included adding draft angles and ensuring uniform part thickness.
I also applied colours to the parts, where blue represents the 5mm dilator, and purple represents the 12mm dilator system, where the cannula itself was kept semi-transparent in order for users to see through (e.g. to look for tissue bleeding).
3-4 iterations of the fully manufactured device were created and tested to meet the functional requirements. For the FDA 510(k) application preparation, we conducted dry runs of our V&V tests.
OUTCOMES

US Patent Issued: As of May 24, 2022, Xpan's US Patent application was granted and published, with a priority date of March 13, 2020.
See: Radially expandable cannula devices, and systems and methods for using them.

FDA 510(k) Application Submitted: As of November 2022, Xpan's FDA 510(k) application has been submitted. We expect clearance in early 2023 at which point Xpan can begin marketing and selling to US hospitals!
KEY TAKEAWAYS
PRODUCT DISCOVERY, PROTOTYPING AND TESTING:
1. Validate the Market Early On
There is no shortage of problems within healthcare, however only few are worth pursuing from a market needs and commercialization perspective. We knew there was a surgical need and innovation gap based on initial conversations with surgeons, however we did not know how big the problem was and which surgical specialties were impacted the most. It was, and should always be a priority to spend the time early on to validate the market prior to diving too deep into the technology.
2. Have a Failure-First Mindset
Product development, especially for medical devices, requires a failure-first mindset. Since we are dealing with products that have direct patient interaction, each feature must be tested to extremes to prevent failure in the field. Failures can range from low-risk malfunctions to high-risk injuries or even patient death. This is very different from a digital app for example, where failures in the field are allowed and even encouraged to identify areas for improvement. Tests should not be designed with the goal of "proving" that a new feature works; but instead designed to "break" the feature by using the worst case scenario.
For example, when testing our valve system design for instrument drag forces, we did so using the worst-case environment; not only real time, but also at several time stamps (e.g. one hour to one year accelerated aging), with various instrument shapes and sizes and with higher insertion cycles (e.g. 50+ instrument insertions until failure). From this, we were able to choose a lubricant that survived the worst case scenario and reduce risk in the field.
3. Avoid Bias & Encourage Storytelling in User Interviews
During problem and market validation, we interviewed and surveyed countless surgeons across various specialties, some of which did not immediately see the value of our solution in their practice upfront. Instead of responding by telling/proving to them how our solution could be useful, we realized the best way was to ask them to "tell us about a time when X happened during Y procedure", or "how often does X happen in Y procecure". These types of questions uncovered gaps and brought their awareness to issues within their practice that they otherwise wouldn't question, and opened up their desire for a solution. Additionally, this process allowed us to further narrow down our target market of surgical specialties that had the greatest need for a solution like ours.
DEVELOPING RELATIONSHIPS:
4. Leverage User Connections
After 200+ cold-call outreach efforts to surgeons (possibly the busiest people on the planet), we were not having much luck. Without an existing, established and trusted brand in the space, we quickly realized the best way to gain more prospective customers at such an early stage was to leverage our existing user base. Surgeons, like most people, highly value the opinions of their colleagues and will generally be more open to a discussion if they are referred as a mutual trust is already in place. Some of our most valuable connections were made through surgeon-surgeon introductions.
5. Be Fluent in Layman's Terms (Translating Technical Language for the General Public)
As an engineer with a technical background, I could go on about the mechanics and complex functionalities built into the product, but ultimately this is not what will convince a user to use it or an investor to invest. The ability to describe in layman's terms how the device addresses a problem at a high level is an important skill to perfect, and is one I continuously work on.
PHOTOS THROUGHOUT THE YEARS




PARTNER ORGANIZATIONS

VIEW MY OTHER PROJECTS:

Xpan Brand Revitalization: Logo & Web DesignDigital Products

New Start Foundation: Logo & Web DesignDigital Products

Fino: A Youth Financial Education AppDigital Products
AR Needle Guidance System for Heart Fluid AspirationPhysical Products

100% Reusable Faceshields for COVID-19Physical Products